JARS v44n1 - The Pigment Constitution Of R. griersonianum
The Pigment Constitution Of R. griersonianum
Kenichi Arisumi, Yusuke Sakata, Sawako Takeshita
Kagoshima University
Kagoshima, Japan
Since its discovery in western Yunnan by George Forrest in 1917, Rhododendron griersonianum has been used extensively in hybridization, largely because of its unusual and distinct red flower color. The study reported here was made to determine the pigment constitution of the flower color of this species in relation to breeding for elepidotes with intense red flower color.
The form of R. griersonianum used in this study was obtained through the courtesy of Mrs. Scott and Mr. Robert Minnich, Puyallup, Washington, in 1986. The pigment constitution of flowers of this form was analyzed by high performance liquid chromatography. The analysis procedures are presented in Figure 1.
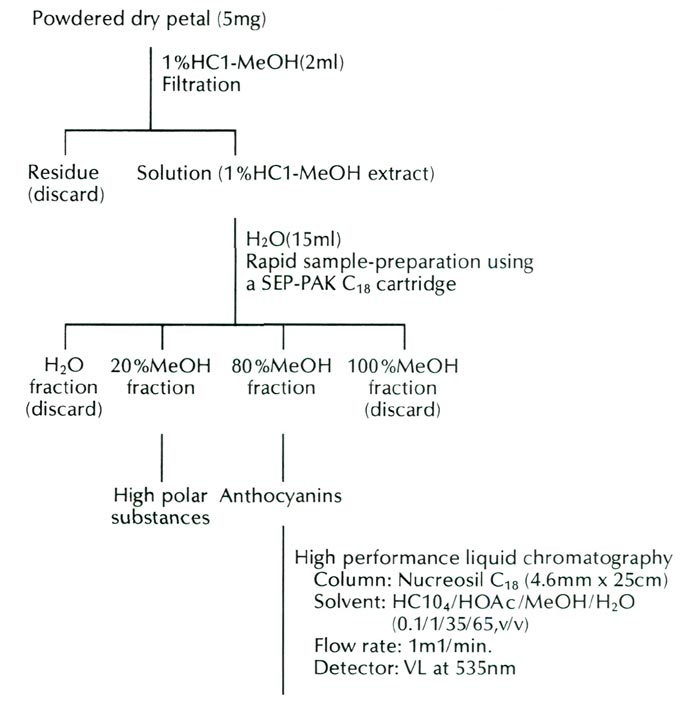
|
| Fig. 1. Procedures of analysis in the anthocyanins of R. griersonianum . |
As shown in Figure 2, the anthocyanins of this species were identified as cyanidin 3-galactoside and cyanidin 3-arabinoside along with a trace amount of cyanidin 3-glucoside, and are compared to the anthocyanins of Camellia japonica ssp. hozanensis collected in Taiwan, which contains cyanidin 3-galactoside, cyanidin 3-glucoside and a small amount of cyanidin 3-arabinoside.
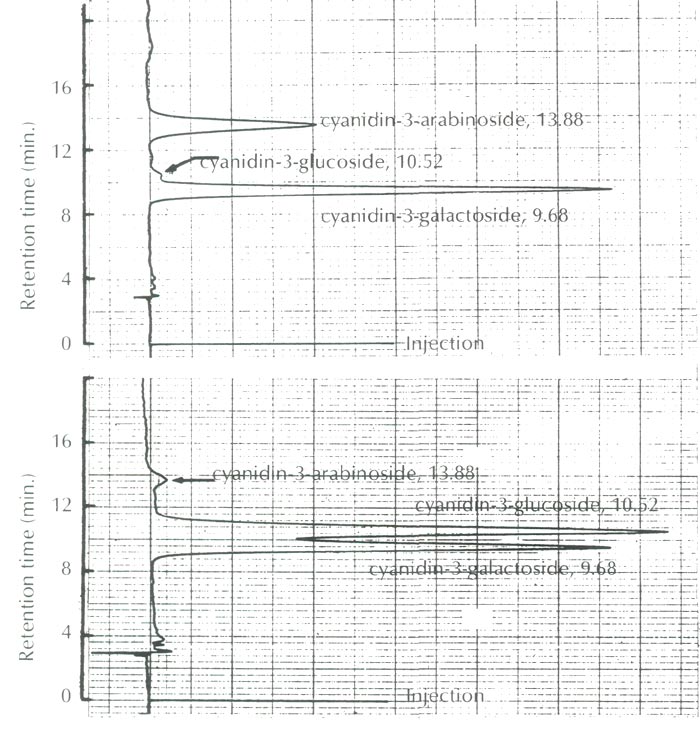
|
|
Fig. 2. HPLC profiles of the anthocyanins of
R. griersonianum
(upper figure)
with the reference anthocyanins of Camellia japonica ssp. hozanensis of Taiwan origin (lower figure). |
Figure 3 shows the result of thin layer chromatography which indicated the presence of a trace amount of an unknown anthocyanin. From its Rf value, higher than those of ordinary 3-glycoside in both organic and aqueous solvent systems, this unknown anthocyanin was deduced to be an acylated anthocyanin of ordinary cyanidin 3-glycoside. However, the amount of the acylated anthocyanin was calculated to be only 0.7% of the total anthocyanins, and thus its effectiveness as a color modifying factor is probably negligible.
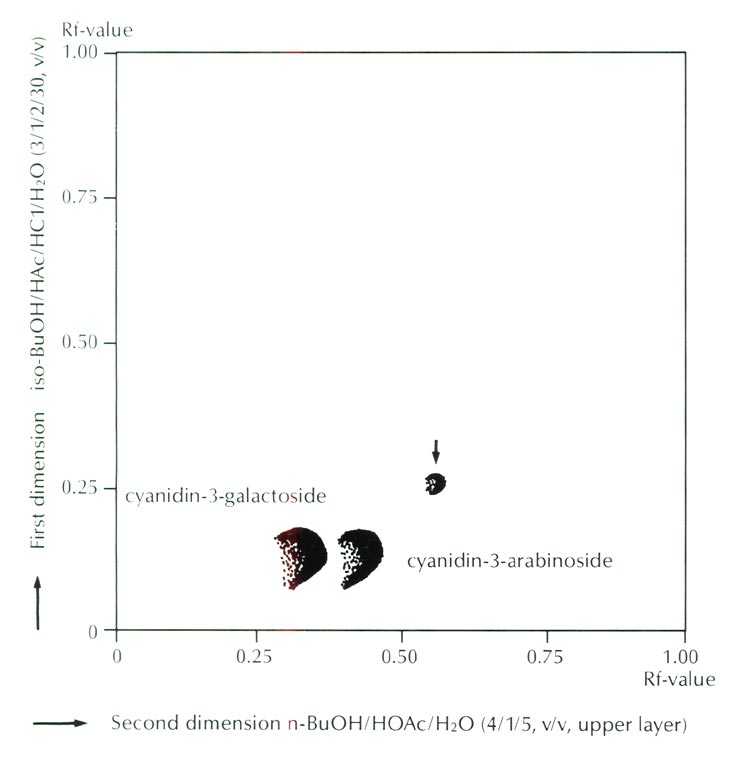
|
|
Fig. 3. Thin layer chromatogram of anthocyanins of
R. griersonianum
.
Arrow indicates an unknown anthocanin. Cyanidin 3-glucoside is included in the spot of cyanidin 3-galactoside. |
Concerning flavonols, many rhododendron and azalea species and cultivars contain myricetin, quercetin and/or kaempferol, frequently together with their related 5-methyl ethers. However, no flavonol was observed in R. griersonianum using our method of analysis. Furthermore, no carotenoid was present, although some unknown pigment which seemed to be the derivative of chlorophyll was detected in very small amounts during the early stages of flowering.
Based on these observations, we have concluded that the unusual and distinct red flower color of R. griersonianum is due mainly to the presence of simple cyanidin 3-glycoside, i.e., the 3-galactoside and 3-arabinoside, free from the mechanism of higher hydroxylation, 5-glycosylation, methylation and acylation of anthocyanin, as well as that of co-pigmentation derived from the coexistence of flavonols. All of these have been known to be factors that blue flower colors, although the intensity of their action is more or less different for each.
In this context, the pigment constitution of R. griersonianum was discovered to be very similar to that of the evergreen azalea of Japanese origin, R. kaempferi . This species contains no flavonol and its anthocyanins are exclusively composed of simple cyanidin 3-glycosides as shown in Figure 4.
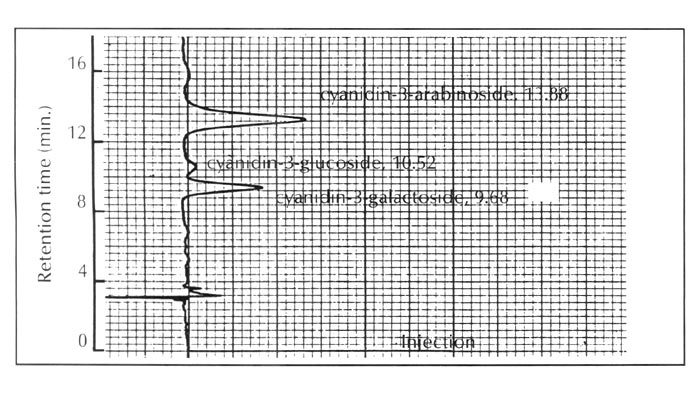
|
| Fig. 4. HPLC profiles of the anthocyanins of R. kaempferi . |
Moreover, in the higher plants inheritance mode, the pigment characteristics mentioned above are known to behave generally in the following fashion. As for the modification of anthocyanins, the relationships of dominant vs. recessive character are: (1) the higher hydroxylation dominant to the lower hydroxylation, (2) the 3:5-diglycosylation to the 3-monoglycosylation, (3) the higher methylation to the lower methylation or the methylation to the non-methylation, (4) the acylation to the non-acylation. The production of flavonol is also known to behave as a dominant characteristic, which is closely related with the formation of co-pigment.
Therefore, the pigment constitution of R. griersonianum should be expected to be genetically recessive. This means also that the realization of intense red hues represented by R. griersonianum requires the systematic re-accumulation of recessive genes, once it has been crossed with other dominant red elepidotes. This can be accomplished by crossing sibling primary hybrids of R. griersonianum or by back crossing.
The authors are associated with the Laboratory of Ornamental Horticulture and Floriculture at Kagoshima University. Kenichi Arisumi and Yusuke Sakata were authors of "Breeding For The Heat Resistant Azaleas — Part I and II", ARS Journal, Vol. 39:4 and Vol. 40:4.
Many thanks to George Ring III, Chairman of the ARS Research Committee, for securing and assisting with the preparation of this article for publication in the ARS Journal.
