JARS v48n4 - A Study of Chromosome Yields and Growth Responses in Colchicine Treated Rhododendrons
A Study of Chromosome Yields and Growth Responses in Colchicine Treated Rhododendrons
John E. Eiselein
El Cerrito, California
Summary
In an effort to increase efficiency of colchicine induced polyploidy, multiple and single exposure modes are compared in rhododendron seedlings. Fractionation of colchicine exposure, interposed with recovery periods, increased chromosome yields. Possible mechanisms are discussed. Results were quantified by direct chromosome count and epifluorescent analysis of chloroplasts in leaf guard cells. Study of colchicine effects are extended to include observations on growth and form. Early growth inhibition, followed by acceleration, are documented, and some abnormal growth forms noted. Mortality, while generally higher, was not progressive with increasing colchicine exposure. Attributing the observed developmental changes to polyploidy is unsupported by results in this study and a search for broader interpretations of somatic effects is suggested.
Introduction
Colchicine, a potent mitotic inhibitor, can induce chromosome doubling in dividing plant cells. This property of genetic potentiation, discovered in 1937, has been exploited over the years to create many improved plant varieties (1).
When rhododendron seedlings or buds are subjected to colchicine, certain characteristic improvement in leaf and flower form develop in some fraction of these plants as they mature. Such changes are assumed a function of induced polyploidy (2), (3), (4).
It is of some practical importance to plant breeders, seeking to produce colchicine improved polyploid hybrids, and researchers, seeking statistically significant effects, to employ treatment techniques designed to optimize chromosome doubling. Unfortunately, there is no consensus on exposure time or concentration of colchicine to guide the workers with any assurances based on quantified data. Concentration of colchicine is reported in thousand-fold differences in the literature ranging from 0.002% to 1.0% (2), (3). Some success on the basis of phenotypic or somatic responses is reported in these methods, but efficiency of chromosome conversion remains undefined.
The objective of this study is to test colchicine exposure time and its effect on the incidence of chromosome doubling based on quantitative analysis of orcein staining of root tip and ultraviolet epifluorescence of leaf chloroplasts.
Materials and Methods
Seedlings of rhododendron hybrids 'Venda Kee'* ('Bruce Brechtbill' x 'Naomi') and 'Gladys Monroe'* ( R. fortunei x R. yakushimanum ) were chosen as study specimens. At their earliest germination the seedlings were exposed on the surface of paper toweling saturated with a 1.0% colchicine solution. This is at the high end of the concentration spectrum for colchicine as reported in the literature.
Fluorescent Gro-lites were place at 18 inches from translucent treatment containers. Light was uninterrupted. Temperature was maintained between 75°F and 85°F. Colchicine exposure was delivered to seedlings in two different dosage patterns.
TREATMENT PATTERN I. Four groups of seedlings received single, uninterrupted colchicine exposure times of 24, 48, 72 or 96 hours respectively.
TREATMENT PATTERN II. A single group of seedlings received four successive 24-hour colchicine exposures, interrupted each exposure time by two- to five-day recovery periods between exposures. Dosage time totaled 96 hours. Colchicine treatments were always followed by large volume tap water rinses of 6-hour duration. See Table 2.
After treatment seedlings were cultivated on fine porosity, water moistened filter paper, held within closed translucent plastic cartons. Illumination was maintained for 16-hour daily intervals at a distance of 18 inches from fluorescent Gro-lites. Room temperature ranged from 60°F to 70°F. After six weeks of cultivation seedling aliquots were either transferred to soil for extended observation or sacrificed for polyploid determinations.
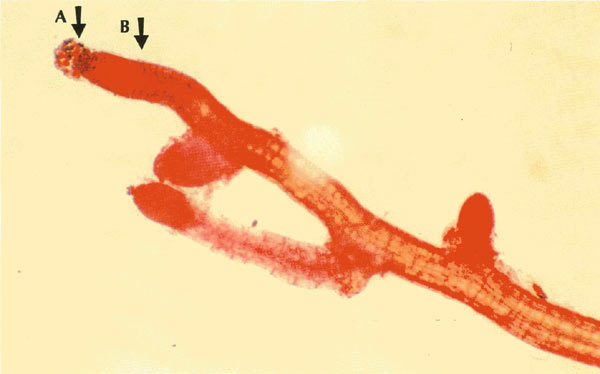
|
|
Figure 1. 200X orcein stained seedling root of 'Gladys Monroe' seedling
six weeks after fractionated colchicine exposure. Note mitotically active ball of loose cells at tip end, Arrow A. These cells, of key interest, are vulnerable to mechanical displacement in staining procedures (5). Arrow B indicates the post-mitotic region where very large chromosome arrays remained in metaphase. Consult Figure 3. Photo by John E. Eiselein |

|
|
Figure 2. 1000X close-up of tetraploid cells budding
at meristematic tip of Figure 1. Photo by John E. Eiselein |
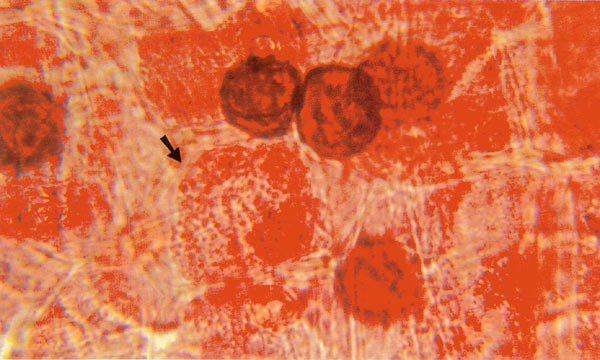
|
|
Figure 3. 1000X. Arrow indicates "megaploid" nuclei at post-mitotic region.
See Arrow B, Figure 1. Cells elongating here following cell division ordinarily present few mitotic figures, but in some fractionated colchicine specimens uncountable numbers of chromosomes are found accumulating in huge nuclei here, seemingly frozen at metaphase. Photo by John E. Eiselein |
Polyploidy was confirmed by direct chromosome count in root tip six weeks after colchicine treatment in seedlings of 'Gladys Monroe' and after a growth period of three years in maturing seedlings of 'Venda Kee', using an orcein staining procedure I presented in detail in an article in the Summer 1994 issue of the ARS Journal (5), or analyzing by counting chloroplasts in leaf guard cells employing an epifluorescent microscopy technique courteously provided by Don Paden, M. M. Meyers and Lane Rayburn of the University of Illinois. Details of this efficient stain-free screening technique are discussed in their 1990 ARS Journal article (6). Figures 4 and 5 are photographs taken of chloroplast bodies through an epifluorescent microscope. The method is fast and very much simpler than staining the tiny stain refractory rhododendron chromosomes. Unfortunately the special epifluorescent microscope, with its special ultraviolet light source, is not commonly available and very expensive to acquire, compared to ordinary light microscopes. Hopefully, this restriction will change as the ultraviolet microscope use becomes more widespread.

|
|
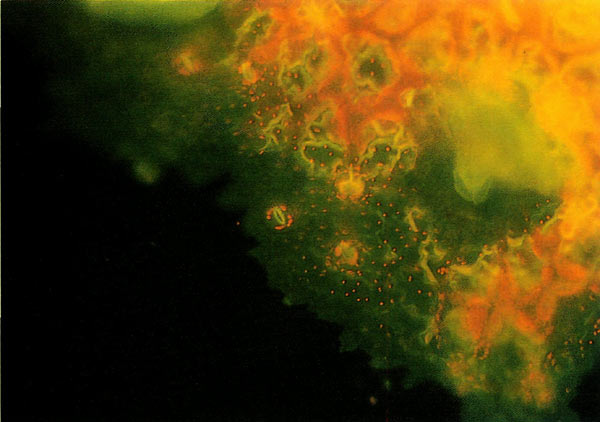
Figure 4. The numbers of chloroplasts glowing in red rosette arrays under
ultraviolet excitation in guard cells of tetraploid seedlings of 'Venda Kee' can be counted. This technique has proven an efficient screening method for polyploidy. Following a lead by Kehr (2) who observed that tetraploid species had larger guard cells than diploid cousins, applying epifluorescent microscopy Paden, Meyers and Rayburn reported several-fold increases in chloroplasts when comparing tetraploid and diploid guard cells in rhododendrons (6). Photo by Paden, Meyers and Rayburn |
|
|
Figure 5. It is easy to discern and count fewer glowing chloroplasts
in this diploid epifluorescent leaf guard display in a control leaf from 'Venda Kee' seedling. Photo by Paden, Meyers and Rayburn |
Results
Chromosome responses were significantly different between single and fractionated exposure modes. Polyploid conversion was not progressive as single exposure times increased from 24 through 96 hours. The response was generally "flat" around 20%. Only 2 of 11 specimens tested as polyploid at the maximum 96-hour exposure time (see Table 1). Comparing this response with the fractionated 96-hour exposure group where 15 or 19 plants (79%) were converted to polyploid the difference was significant. P = less than 0.01.
| Table 1: 'Gladys Monroe' Seedlings | |||
| Exposure | Poly/Diploid | % Conversion | Six Weeks Dead/Alive |
| 0 hrs. | 1/25 | ||
| 24 hrs. | 3/12 | 25% | 3/25 |
| 48 hrs. | 2/11 | 18% | 4/27 |
| 72 hrs. | 2/10 | 18% | 3/20 |
| Table 2: 'Gladys Monroe' Seedlings | ||||
| Treatment Fractionation Scheme | ||||
| Treatments | Time | Recovery Time | Poly/Diploid | % Conversion |
| No. 1 | +24 hrs. | 2 days | ||
| No. 2 | +24 hrs. | 5 days | ||
| No. 3 | +24 hrs. | 2 days | ||
| No. 4 | +24 hrs. | 2 days | ||
| +96 hrs. total treatment time | 15/19 | 79% | ||
We should expect in cell populations of fractionated exposure groups that chromosome complements be mixoploid. Indeed, it was discovered that 4 of the 15 polyploids, while presenting predominately tetraploid counts, also contained massive, uncountable "megaploid" chromosome arrays. Such an interesting field of chromosomes can be seen in the photomicrograph, Figure 3.
Discussion
How do we explain the increased conversion efficiency by fractionating colchicine exposure? It is known that one effect of colchicine is disruption of the mitotic fibers formed at metaphase, preventing the chromosomes that have been duplicated during prophase from being drawn apart into two new daughter nuclei. Remaining un-separated, the nucleus retains a doubled chromosome array. When colchicine is withdrawn and prophase again proceeds to duplicate the chromosomes, and metaphase spindle fibers are allowed to function intact, tetraploid chromosome complements are drawn into two new daughter cells. Polyploidy is thus born.
If we hypothesize a broader mitotic effect, namely that colchicine blocks resting cells from entering prophase, and assume further that only 20 percent of the meristematic cell populations is in metaphase synchrony at any one time, colchicine would target these cells, as well as interrupt prophase until such time as colchicine is withdrawn. If colchicine exposure is terminated then roughly 20 percent of the dividing cell population would end as tetraploids, as was determined in the single exposure mode of this study. Allowing a suitable recovery period, however, mitotic processes would reactivate, providing another estimated 20 percent colchicine sensitive cell fraction. Given this scenario the mixoploid cell population— tetraploid, octoploid, "megaploid" and so on—would thus expand with each successive colchicine application.
Developmental Effects
In addition to the conversion efficiency study, an ancillary group was treated with 1.0% colchicine for 24 hours and control seedlings were set in the soil for continued observation over time for any notable developmental differences and their possible correlation with polyploidy. From the start, growth rate was severely inhibited in the seedlings of 'Venda Kee'. After six months of cultivation 100% of the colchicine treated specimens remained almost embryonic in appearance, measuring around 1.5 cm, retaining primary leaves and underdeveloped roots. The striking effect can be seen in the Figure 6 photograph.
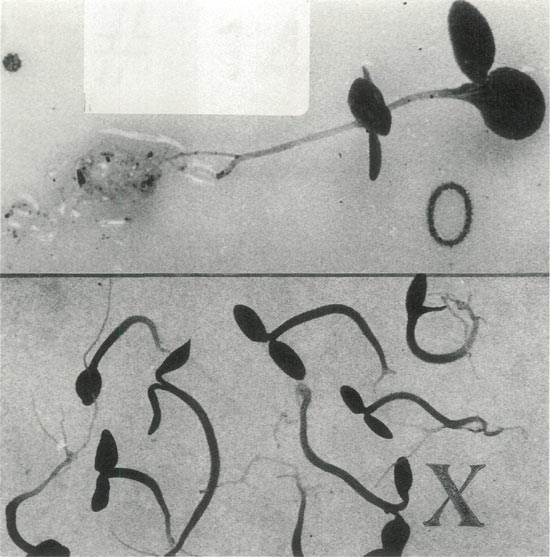
|
|
Figure 6. Colchicine inhibits early growth in rhododendrons.
An extreme response is seen here in a 'Venda Kee' seedling six months after treatment. The seedlings grouped in the lower section remain severely underdeveloped when compared with the representative control plant extending across the top of the photo. Photo by John E. Eiselein |
From my observations on colchicine effects in other hybrids and species the inhibition is generally more moderate, as was the case with seedlings of 'Gladys Monroe', as demonstrated at six months in the field planted specimens shown in Figure 7.

|
|
Figure 7. Growth inhibition was moderate in the case of
'Gladys Monroe' seedlings response to colchicine. These planted specimens have grown for 6 months since treatment. First row, bottom, are controls and the top three rows are experimental plants. Photo by John E. Eiselein |
In seedlings of 'Venda Kee', following the period of inhibition, growth accelerated, and at least half of the diminutive colchicine plants achieved growth parity with the controls. The other half of the group, however, remained significantly smaller. Survivors were few in both experimental and control groups. Only 12 of 150 colchicine treated plants and 13 of 100 controls survived three years. Although higher in the experimental group, the difference in mortality was not statistically significant. Figure 8 is a photo of two normal sized experimental plants with two representative control plants. The diminutive group of six experimental plants was more provocative in appearance. Not only were they small, measuring only two or three inches tall, five of them had short, heavy textured, drooping leaves. The overall impression of the group was that of misshapen dwarfs. They can be seen in Figure 9. None of the control plants appeared abnormal.

|
|
Figure 8. 'Venda Kee' seedlings, so severely retarded at 6 months
(shown here at 3 years), were stimulated into accelerating growth, reaching size parity with controls by one year. Plants numbered 1 and 4 are controls, and plants numbered 2 and 3 are colchicine treated. Number 3 is a tetraploid. There is nothing to distinguish any polyploid plant from the other diploids at this time. If polyploid is responsible for attributable somatic changes, we would expect, as these plants mature, such effects would ultimately be expressed. Photo by John E. Eiselein |
From my observations on colchicine effects in other hybrids and species the inhibition is generally more moderate, as was the case with seedlings of 'Gladys Monroe', as demonstrated at six months in the field planted specimens shown in Figure 7.
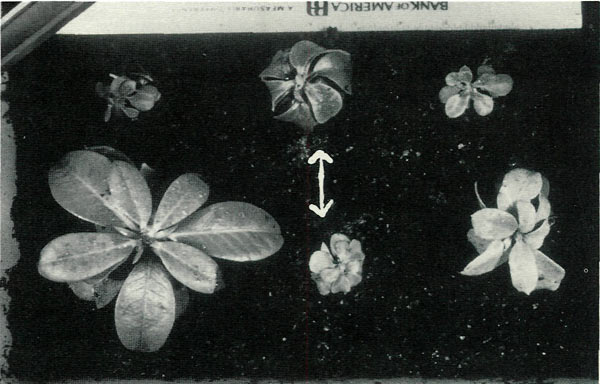
|
|
Figure 9. Six of 12 surviving colchicine treated 'Venda Kee'
seedlings did not attain normal stature even by three years as shown here. It can be seen that they are dwarfs with thick, short, down-curling leaves. The effect is not due to polyploidy. Only the center two plants are tetraploid, as determined both by epifluorescent screening and direct root cell chromosome count. Tetraploids are indicated by arrows. Photo by John E. Eiselein |
One might well assume, as I did at the time, that all six of the tiny plants were polyploids, but this was not to be. Testing for polyploidy by epifluorescent screening and direct chromosome counting revealed only two of the six dwarfs were polyploids. The same ratio of plants tested polyploid among the six normal sized colchicine treated group, namely, two of six, and at this time there is no somatic effects to distinguish them from controls.
It has to be concluded from these observations, even though the numbers are small, that the developmental differences involving growth rates and dwarfism are not reflections of induced polyploidy in specimens in this study.
I believe we should consider other effects of colchicine treatment in addition to changes in chromosome numbers; these include hormonal and chemical changes, point mutations, changes in chromosomal organelles, changes in chloroplast bodies, and changes in other genetic and physiological entities. The complexity of colchicine effects on physiology and chemistry are well documented in food crops and flowers, and such effects should be explored in high-order woody plants such as rhododendrons, especially as they may relate to somatic enhancement associated with colchicine treatment (1), (7). Without nuclear confirmation, however, we should be careful not to assume such effects are always chromosomal. Improvements in staining techniques using orcein, coupled with methods increasing chromosome yields to improve statistics, will, I hope, implement more accurate studies of rhododendron responses to colchicine. There is much challenging work that can be done with this approach.
Acknowledgments
The author greatly appreciates the contributions of August Kehr, for his invaluable council, and Don Paden and Lane Rayburn for their generous analysis of chloroplasts in the leaf specimens.
Dedication
This study is dedicated to the memory of Gladys M. Eiselein and Venda B. Kee.
References
1. Eigsti, O. J.; Dustin, P. Colchicine in agriculture, medicine, biology, chemistry. Ames, IA: Iowa University Press; 1955.
2. Kehr, A. E. A tetraploid
Rhododendron carolinianum
. ARS Bulletin, 25:4-7; 1971.
3. Leach, D. G. Rhododendrons of the world. New York: Charles Scribner's Sons; 1961.
4. Pryor, R. I; Frazier, L. C. Colchicine induced tetraploid azaleas. Hort. Science, 3: 383-385; 1968.
5. Eiselein, J. E. An improved staining method applied to the study of colchicine effects in
Rhododendron
. J. Amer. Rhod. Soc., 48:143-146; 1994.
6. Paden, D. W.; Meyers, M. M., Jr.; Rayburn, A. L. Doubling chromosomes with cholchicine treatment invitro as determined by chloroplast numbers in epidermal guard cells. J. Amer. Rhod. Soc. 44: 162-171; 1990.
7. Tilney-Basset, R. A. E. Plant chimeras. London: Edward Arnold; 1986.
John E. Eiselein is a research scientist at the University of California, Lawrence Livermore Laboratory, Livermore, Calif.
